Understanding the Differences
Learn the differences between oral MTX and injectable MTX, such as an auto-injector or a syringe and vial option.
 |
 |
 |
|
| Oral MTX | Auto-injector MTX | Syringe and vial MTX | |
|
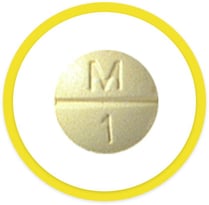
A pill taken by mouth
|
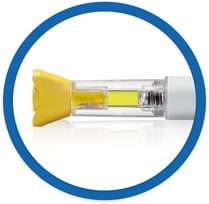
A prefilled single-dose device that allows the drug to be injected manually under the skin
|
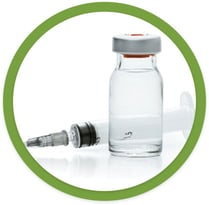
MTX liquid drawn from a vial into a single-use syringe that can then be injected manually under the skin
|
|
 Ease of use Ease of use |
Taken by mouth; swallowed with liquid
|
Prefilled and ready to use, requires fewer steps than a vial and syringe
|
More steps are required than with a pill or with an auto-injector. The drug must be precisely measured and drawn into a single-use syringe before the needle can then be injected manually under the skin.
|
|
Yes
|
Yes
|
Yes. However, more items are required for administration than with oral MTX or with an auto-injector.
|
|
|
N/A
|
The needle shield on the Rasuvo ® (methotrexate) injection device minimizes the potential for needle injuries.
|
The needle of the syringe is exposed, increasing the potential for needle injuries.
|
MTX is absorbed differently depending on the delivery
Drugs need to be absorbed into the body before they can go where they are needed. Oral drugs are absorbed through your stomach and small intestine. As a result, the full drug dose may not be absorbed into the body.
Drugs that are injected under the skin—such as Rasuvo—do not have to go through the stomach to be absorbed.
Why is this important? Basically, patients may experience fewer gastrointestinal (GI) side effects and have a better response to treatment with Rasuvo than with oral MTX. A recent study showed that healthy people who were given Rasuvo absorbed more MTX than did those who were given MTX tablets. In this study, people who were given Rasuvo had fewer GI side effects.
This information doesn’t guarantee that Rasuvo will work better and have fewer GI side effects in patients. However, it may explain why some patients taking Rasuvo experience greater control of symptoms and fewer GI side effects than patients taking oral MTX. Talk to your doctor about Rasuvo to see if it is right for you.
Download a patient brochure to learn more.

What is Rasuvo® (methotrexate) injection?
Rasuvo is a single-dose auto-injector containing a prescription medicine, methotrexate. Methotrexate is used to:
- Treat certain adults with severe, active rheumatoid arthritis, and children with active polyarticular juvenile idiopathic arthritis (pJIA), after treatment with other medicines including non-steroidal anti-inflammatory (NSAIDS) have been used and did not work well.
- Control the symptoms of severe, resistant, disabling psoriasis in adults when other types of treatment have been used and did not work well.
Rasuvo should not be used for the treatment of cancer.
Rasuvo should not be used for the treatment of children with psoriasis.
Rasuvo is available in doses of 7.5, 10, 12.5, 15, 17.5, 20, 22.5, 25, and 30 mg. Your doctor will prescribe a different way to take methotrexate if you need to take methotrexate by mouth or in some other way.
Important Safety Information, Including Boxed Warning, for Rasuvo
What is the most important information I should know about Rasuvo?
This product includes the following Boxed Warning:
Rasuvo can cause serious side effects that can lead to death, including:
Organ system toxicity. People who use methotrexate for the treatment of cancer, psoriasis, or rheumatoid arthritis, have an increased risk of death from organ toxicity. Types of organ toxicity can include: gastrointestinal, nerve, bone marrow, lung, liver, kidneys, immune system, and skin.
Your doctor will do blood tests and other types of tests before you take and while you are taking Rasuvo to check for signs and symptoms of organ toxicity. Call your doctor right away if you have any of the following symptoms of organ toxicity: vomiting, neck stiffness, diarrhea, paralysis, mouth sores, irritability, fever, sleepiness, confusion, problems with coordination, weakness, dry cough, temporary blindness, trouble breathing, seizures, severe skin rash, headache, and back pain.
Women who are pregnant are at increased risk for death of the baby and for birth defects in the baby. Women who are pregnant or who plan to become pregnant must not take Rasuvo. A pregnancy test should be performed before starting Rasuvo. Contraception should be used by both females and males while taking Rasuvo. Pregnancy should be avoided if either partner is receiving Rasuvo:
- for a minimum of 3 months after treatment with Rasuvo for males.
- during and for at least 1 menstrual cycle after treatment with Rasuvo for females.
What are the possible side effects of Rasuvo?
Rasuvo may cause serious side effects, including:
- Fertility problems. Methotrexate, the active ingredient in Rasuvo, may affect your ability to have a baby. Males may have a decreased sperm count, and females may have changes to their menstrual cycle. This can happen while taking Rasuvo and for a short period of time after you stop.
- Certain cancers. Some people who have taken methotrexate have had a certain type of cancer called Non-Hodgkin’s lymphoma and other tumors. Your doctor may tell you to stop taking Rasuvo if this happens.
- Tissue and bone problems. Taking methotrexate while having radiation therapy may increase the risk of your tissue or bone not receiving enough blood. This may lead to death of the tissue or bone.
Common side effects of Rasuvo include: nausea, stomach pain, indigestion (dyspepsia), mouth sores, and rash.
Who should not take Rasuvo?
Do not take Rasuvo if you:
- Are pregnant or planning to become pregnant
- Are breastfeeding; Rasuvo can pass into your breast milk and may harm your baby.
- Have alcohol problems (alcoholism)
- Have liver problems
- Have problems fighting infection (immunodeficiency syndrome)
- Have been told you have (or think you have) a blood disorder, such as low levels of white blood cells, red blood cells (anemia), or platelets
- Have had an allergy to methotrexate or any of the ingredients in Rasuvo
What should I tell my doctor before taking Rasuvo?
Before you take Rasuvo, tell your doctor if you have any other medical conditions. Tell your doctor about all of the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
Rasuvo may affect how other medicines work, and other medicines may affect how Rasuvo works, causing side effects. Ask your doctor or pharmacist for a list of medicines if you are not sure.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Rasuvo. For more information, ask your doctor or pharmacist.
For additional information about Rasuvo, please refer to the Patient Information Leaflet and Instructions for Use, which can be found at www.rasuvo.com or call 1-855-336-3322.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.

